Occlutech is a leading specialist provider of minimally invasive cardiac devices
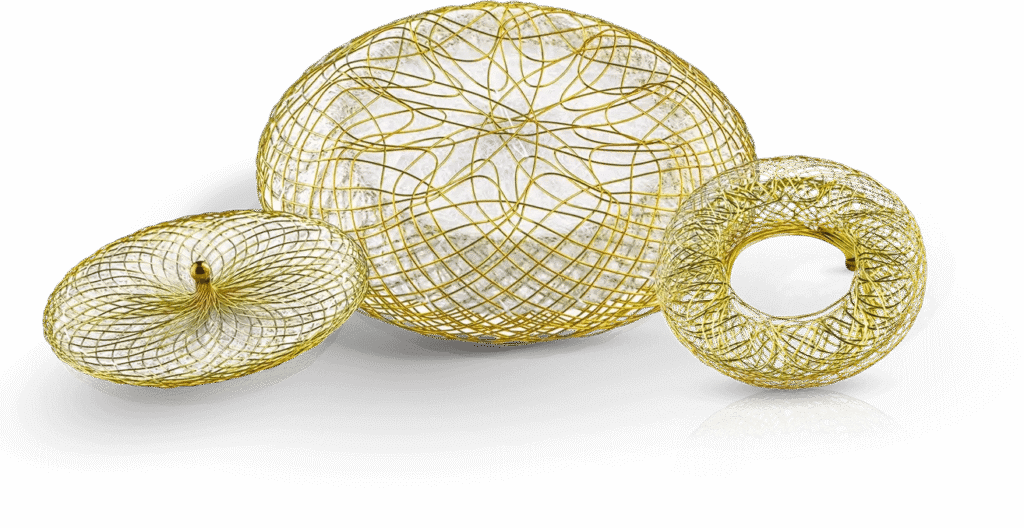
Our mission is to improve the quality of life for people with heart conditions, collaborating with leading healthcare professionals to design, manufacture, and commercialize best in class cardiac devices.
Our products make a life changing difference.
Driven by a passion for innovation, Occlutech has developed, manufactured, and commercialized minimally invasive cardiac implants since 2003. We offer a broad and proven portfolio designed to address Congenital Heart Disease, Stroke Prevention & Adult Intervention, and Heart Failure.
Over 205 000 occluders
in over 70 countries
Occlutech’s products are implanted in thousands of human hearts every year. Quality and the product’s consistent performance are key to the trust of thousands of physicians who use them, because, every beat counts.
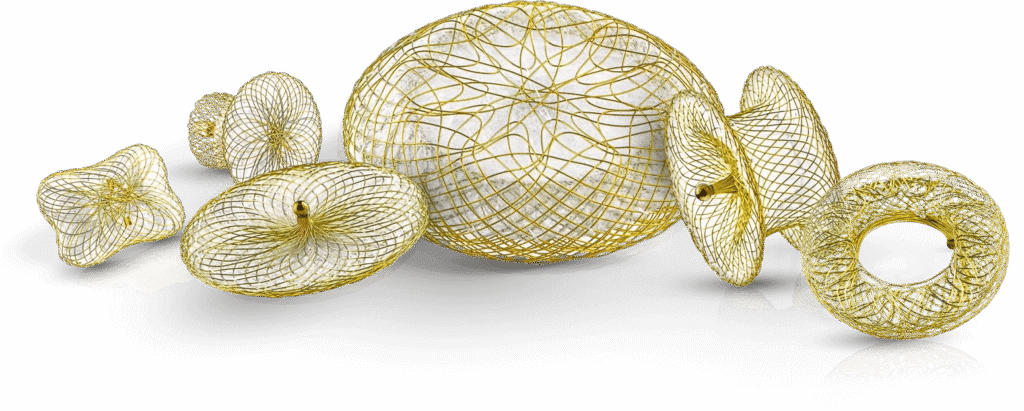
We are proudly assisting those who save lives
#occlutech #everybeatcounts